New podcast episode!
Last week Sam and I sat down with our good friend Aaron Andersen, who recently graduated with a BS in Food Science, to talk to him about food, preservatives, eggs, and synthetic meat. Enjoy!
Show Notes:
Topic; Food Science
Panel: Chad Jones, Sam Matthews, and Aaron Andersen
Thursday, August 22, 2013
Sunday, August 18, 2013
Chemistry from an outsiders perspective
Fellow chemistry bloggers/enthusiasts: Let's take a look at this whole "chemistry doesn't get any love" thing the best way we can. I would like to officially submit the following paper for peer review (that is, for review by my chemblog peers). Please understand before you read the sarcastic nature of my writing in this format...
Abstract
This article is presented (at least in part) as a tongue-in-cheek look at the public's perspective of chemistry. Chemistry bloggers often remark that chemistry is poorly featured in mainstream science communication. To assess the validity of this claim a Google search results experiment was performed. This experiment showed that chemistry fairs just about as well as physics and biology. To further test the claim, content on the social media webpage "I F***ing Love Science" was analyzed and categorized by discipline. This study shows a high bias towards biology and astronomy on IFLS.
Introduction
Whether or not the chemistry is a well appreciated science among the general public is a topic of recent debate among the online chemistry community. Oh et al. have pointed out [1] that no chemists are mentioned in WIRED Magazine's "101 Signals", which lists the "...best reporters, writers, and thinkers on the Internet". This oversight has restarted a common theme among chemistry bloggers: How is chemistry perceived by the general public?[2][3][4][5]
The experiment herein described was performed as an attempt to quantitatively answer that question. Specifically, it has been designed to test the accessibility and visibility of chemistry as compared to other disciplines. For the purposes of this study "accessible" is defined as "perceived as being easily understood" and "visible" is defined as "featured often in popular culture". Thus a discipline that is both accessible and visible is one that is seen often by the public and is thought of as being easy to understand. The results of this study should be used to shape the future actions of chemistry advocates. If chemistry as a discipline is indeed seen as inaccessible to the general public then specific efforts are needed to make topics in chemistry more accessible. On the other hand, if the science is accessible but not visible then efforts should be focusing on spreading chemistry as it is already presented.
Methods
To assess the public's opinion of chemistry as a whole a Google search experiment was designed. This experiment is designed to take a comparative look on how chemistry is perceived. First, negative search phrases were used for a variety of science disciplines (physics, chemistry, biology, math, and astronomy). The number of search results for each discipline were recorded and normalized to represent a percentage of total search results. Next, positive search phrases were used for the same disciplines. The data were normalized in a similar fashion. Error bars on each result represent a single standard deviation from the average percentage of total search results. While simply counting the number of search results does not perfectly correlate to how people feel about a given subject, it is a good first approximation.
Because the author is a masochist, a second experiment was performed to compare to Google search results. The last 117 posts of the popular science blog "I F***ing Love Science" (IFLS) were categorized as being about Physics, Chemistry, Biology, Math, Astronomy, or other. For counting purposes, jokes about chemistry were considered chemistry content, jokes about physics were considered physics content, etc.
Results and discussion
Figure 1 shows the distribution of negative search results. If chemistry were seen as an inaccessible subject one would expect chemistry to have a higher percentage of negative search results. Instead we see that chemistry, physics, and biology all have about the same number of negative search results while astronomy has a very low negative search result. Math has a very high negative search results. This places math as a very inaccessible discipline.
Figure 2 shows the distribution of positive search results. If chemistry were not a visible discipline one would expect low positive results. These results, however, show that chemistry is as popular as physics and astronomy. Biology is slightly less popular while math is slightly more popular. It is interesting to notice that math scored high in both positive and negative search results.
Figure 3 plots the percentage of content on IFLS with respect to discipline. It is automatically apparent that IFLS shows a high bias to biology and astronomy while featuring very little physics, math, and chemistry by comparison.
While IFLS is by no means a perfect snapshot of the public's interest, it is a reasonable representation of how an educated general audience feels about science. It should be stated that IFLS has a very large audience. Pictures and comments on the site are spread widely throughout Facebook and other social media. It would be in the interest of chemistry advocates to appeal to Elise Andrew, author of the blog, for more chemistry content.
Conclusion
The author of this study readily admits its limitations. It should not be seen as a final say in this discussion. Instead its purpose is to bring a quantitative look at a real problem. Our goal, as science communicators, should be to make our discipline both accessible and visible.
Acknowledgments: The author would like to acknowledge the irony of contributing such a formal article to the discussion about the accessibility of online chemistry education . . .
As this article is being submitted for peer review, I welcome criticisms that go along with that process. If you have new search phrases you think should be included or any other modifications please comment and I will update the results.
A study of Google search results to understand the public's perspective of chemistry
Chad Jones
The Collapsed Wavefunction
Chad Jones
The Collapsed Wavefunction
Abstract
This article is presented (at least in part) as a tongue-in-cheek look at the public's perspective of chemistry. Chemistry bloggers often remark that chemistry is poorly featured in mainstream science communication. To assess the validity of this claim a Google search results experiment was performed. This experiment showed that chemistry fairs just about as well as physics and biology. To further test the claim, content on the social media webpage "I F***ing Love Science" was analyzed and categorized by discipline. This study shows a high bias towards biology and astronomy on IFLS.
Introduction
Whether or not the chemistry is a well appreciated science among the general public is a topic of recent debate among the online chemistry community. Oh et al. have pointed out [1] that no chemists are mentioned in WIRED Magazine's "101 Signals", which lists the "...best reporters, writers, and thinkers on the Internet". This oversight has restarted a common theme among chemistry bloggers: How is chemistry perceived by the general public?[2][3][4][5]
The experiment herein described was performed as an attempt to quantitatively answer that question. Specifically, it has been designed to test the accessibility and visibility of chemistry as compared to other disciplines. For the purposes of this study "accessible" is defined as "perceived as being easily understood" and "visible" is defined as "featured often in popular culture". Thus a discipline that is both accessible and visible is one that is seen often by the public and is thought of as being easy to understand. The results of this study should be used to shape the future actions of chemistry advocates. If chemistry as a discipline is indeed seen as inaccessible to the general public then specific efforts are needed to make topics in chemistry more accessible. On the other hand, if the science is accessible but not visible then efforts should be focusing on spreading chemistry as it is already presented.
Methods
To assess the public's opinion of chemistry as a whole a Google search experiment was designed. This experiment is designed to take a comparative look on how chemistry is perceived. First, negative search phrases were used for a variety of science disciplines (physics, chemistry, biology, math, and astronomy). The number of search results for each discipline were recorded and normalized to represent a percentage of total search results. Next, positive search phrases were used for the same disciplines. The data were normalized in a similar fashion. Error bars on each result represent a single standard deviation from the average percentage of total search results. While simply counting the number of search results does not perfectly correlate to how people feel about a given subject, it is a good first approximation.
Negative search phrases used:
"...is stupid"
"I hate…"
"…is wrong"
"…is hard"
"I failed…"
"I can't stand…"
"I don't like…"
"…is dumb"
"I'm too stupid for…"
"I don't understand…"
"…isn't useful"
"…is dangerous"
Positive search phrases used:
"…is awesome"
"…is great"
"…is fun"
"…is useful"
"…is my favorite
"I love…"
"I like…"
"…is the best"
Results and discussion
Figure 1 shows the distribution of negative search results. If chemistry were seen as an inaccessible subject one would expect chemistry to have a higher percentage of negative search results. Instead we see that chemistry, physics, and biology all have about the same number of negative search results while astronomy has a very low negative search result. Math has a very high negative search results. This places math as a very inaccessible discipline.

|
| Figure1 - Distribution of negative search results |
Figure 2 shows the distribution of positive search results. If chemistry were not a visible discipline one would expect low positive results. These results, however, show that chemistry is as popular as physics and astronomy. Biology is slightly less popular while math is slightly more popular. It is interesting to notice that math scored high in both positive and negative search results.

|
| Figure 2 - Distribution of positive search results |
Figure 3 plots the percentage of content on IFLS with respect to discipline. It is automatically apparent that IFLS shows a high bias to biology and astronomy while featuring very little physics, math, and chemistry by comparison.
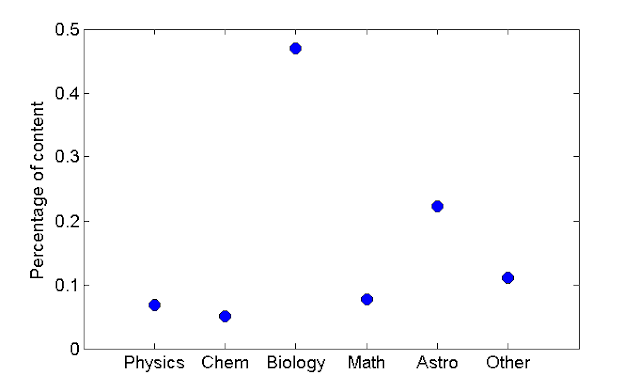
|
| Figure 3 - IFLS content |
While IFLS is by no means a perfect snapshot of the public's interest, it is a reasonable representation of how an educated general audience feels about science. It should be stated that IFLS has a very large audience. Pictures and comments on the site are spread widely throughout Facebook and other social media. It would be in the interest of chemistry advocates to appeal to Elise Andrew, author of the blog, for more chemistry content.
Conclusion
The author of this study readily admits its limitations. It should not be seen as a final say in this discussion. Instead its purpose is to bring a quantitative look at a real problem. Our goal, as science communicators, should be to make our discipline both accessible and visible.
Acknowledgments: The author would like to acknowledge the irony of contributing such a formal article to the discussion about the accessibility of online chemistry education . . .
As this article is being submitted for peer review, I welcome criticisms that go along with that process. If you have new search phrases you think should be included or any other modifications please comment and I will update the results.
Thursday, August 15, 2013
Significant figures
I recently wrote another article for my blog at chem.answers.com about significant figures.
For some reason this simple topic - one that is covered over and over in general chemistry classes - is one that really interests me. I love thinking of new analogies for why sig figs are important, and oddly enough I really liked writing this article.
Chemistry friends please leave a comment or a response on Twitter. I want to know:
For some reason this simple topic - one that is covered over and over in general chemistry classes - is one that really interests me. I love thinking of new analogies for why sig figs are important, and oddly enough I really liked writing this article.
Chemistry friends please leave a comment or a response on Twitter. I want to know:
What is a general chemistry topic that you never get sick of hearing or talking about?
Mine is obviously significant figures....
Wednesday, August 14, 2013
Well Intentioned Bad Science is Still Bad Science
I recently saw an interesting anti-smoking ad:
Let's see what Picard thinks of this ad:

That's what I thought. You see, molecules aren't miniature monsters with eyes and teeth. I understand why they used that imagery; they want to show how frightening these chemicals can be. I can't really call this chemophobia, because it would not be irrational to avoid putting these chemicals in your body.
This public service announcement is doing a significant public disservice, though. People are highly influenced by what they see on TV. This ad is misinforming viewers on the true nature of chemistry. Worst of all, it doesn't need to be done. Why not show the actual molecules? I'd even be ok with a little anthropomorphism; make the actual molecule look menacing. But well intentioned bad science is still bad science.
Let's see what Picard thinks of this ad:

That's what I thought. You see, molecules aren't miniature monsters with eyes and teeth. I understand why they used that imagery; they want to show how frightening these chemicals can be. I can't really call this chemophobia, because it would not be irrational to avoid putting these chemicals in your body.
This public service announcement is doing a significant public disservice, though. People are highly influenced by what they see on TV. This ad is misinforming viewers on the true nature of chemistry. Worst of all, it doesn't need to be done. Why not show the actual molecules? I'd even be ok with a little anthropomorphism; make the actual molecule look menacing. But well intentioned bad science is still bad science.
Tuesday, August 13, 2013
A Chemist's Thoughts on the MythBuster's Breaking Bad Special
I love Breaking Bad. I've watched each episode as it airs since episode #1. I started watching because of the chemistry. So I was excited to see
HF is an extremely nasty acid, don't let the MythBuster's episode fool you into thinking you won't regret a splash of HF (believe me, I've experienced one). One of the real dangers of HF can be described using the principles that Adam taught.
That explanation by Adam was probably the best chemistry related scenes I've seen on television in a long time. It's great to see chemistry on national television in a positive light. Adam's explanation was both accurate and entertaining and I loved it.
That being said, I think they missed out by not explaining the reason that HF is so dangerous. This may sound counter-intuitive, but it's precisely because it is a weak acid that HF can kill you without warning. As Adam said, HF doesn't release a lot of hydrogen atoms. This means that you can be "burned" by HF without even realizing it. There is a small amount of fluorine released which can bind to calcium in your blood and bones. This releases even more fluorine (Le Chatelier's principle) which binds to more calcium in your bones and blood. Even small HF splashes can lead to death by robbing your body of necessary calcium. HF can lead to a host of health problems, so please don't assume that part of the myth is busted.
I wish the MythBusters would have been more thorough with their HF testing. They were quite thorough testing their "special sauce", but we only got to see 100 mL added to a small sample of meat, steel, linoleum, wood, ceramic, and drywall. Here's the thing: HF is known to etch glass. That's the entire premise for the Breaking Bad episode. The MythBusters test fiberglass later in the episode, why not earlier with the HF? I would have loved to see a full scale HF test, and I think the MythBusters missed the mark by not doing it. It may have taken some extra time to work, but I'm certain a pig, lots of HF, and a fiberglass tub would have gotten closer to a Breaking Bad scene recreation. Sure the pig wouldn't be completely dissolved, but neither was the body.
Instead of a full scale HF test the MythBusters decided to change the acid they were using. Now, the MythBusters wisely chose not to divulge what they used to actually dissolve a body. That's not really the sort of education they're interested in giving, I assume. They did give a few hints about the acid they were using and it should have been clear to most chemists that the nasty "special sauce" they used would indeed eat through a pig like a tub full of piranhas - which it did. I thought the full scale test of their "special sauce" was great. They failed to make one connection, though. The myth of easily dissolving a body in acid is busted because that process is extremely exothermic. Just look at the steam rising from this acid bath.
The mercury fulminate scene was the other myth they tested, and it didn't fare well at all. The myth was easily busted, which is a result I would have expected given the extreme nature of the myth. To defend my favorite show, though, the science was exaggerated as a plot device. As I said in our most recent podcast with See Arr Oh, I think exaggerated science is fine as long as it's necessary for the plot and not too unbelievable.
In the end I give this episode of MythBusters an A-. The only thing I think it as missing was a better treatment of what HF can actually do. They did an excellent job of explaining acid chemistry, which was nice.
HF is an extremely nasty acid, don't let the MythBuster's episode fool you into thinking you won't regret a splash of HF (believe me, I've experienced one). One of the real dangers of HF can be described using the principles that Adam taught.

|
| A very happy moment for anyone interested in chemical education |
That explanation by Adam was probably the best chemistry related scenes I've seen on television in a long time. It's great to see chemistry on national television in a positive light. Adam's explanation was both accurate and entertaining and I loved it.
That being said, I think they missed out by not explaining the reason that HF is so dangerous. This may sound counter-intuitive, but it's precisely because it is a weak acid that HF can kill you without warning. As Adam said, HF doesn't release a lot of hydrogen atoms. This means that you can be "burned" by HF without even realizing it. There is a small amount of fluorine released which can bind to calcium in your blood and bones. This releases even more fluorine (Le Chatelier's principle) which binds to more calcium in your bones and blood. Even small HF splashes can lead to death by robbing your body of necessary calcium. HF can lead to a host of health problems, so please don't assume that part of the myth is busted.
I wish the MythBusters would have been more thorough with their HF testing. They were quite thorough testing their "special sauce", but we only got to see 100 mL added to a small sample of meat, steel, linoleum, wood, ceramic, and drywall. Here's the thing: HF is known to etch glass. That's the entire premise for the Breaking Bad episode. The MythBusters test fiberglass later in the episode, why not earlier with the HF? I would have loved to see a full scale HF test, and I think the MythBusters missed the mark by not doing it. It may have taken some extra time to work, but I'm certain a pig, lots of HF, and a fiberglass tub would have gotten closer to a Breaking Bad scene recreation. Sure the pig wouldn't be completely dissolved, but neither was the body.
Instead of a full scale HF test the MythBusters decided to change the acid they were using. Now, the MythBusters wisely chose not to divulge what they used to actually dissolve a body. That's not really the sort of education they're interested in giving, I assume. They did give a few hints about the acid they were using and it should have been clear to most chemists that the nasty "special sauce" they used would indeed eat through a pig like a tub full of piranhas - which it did. I thought the full scale test of their "special sauce" was great. They failed to make one connection, though. The myth of easily dissolving a body in acid is busted because that process is extremely exothermic. Just look at the steam rising from this acid bath.
The mercury fulminate scene was the other myth they tested, and it didn't fare well at all. The myth was easily busted, which is a result I would have expected given the extreme nature of the myth. To defend my favorite show, though, the science was exaggerated as a plot device. As I said in our most recent podcast with See Arr Oh, I think exaggerated science is fine as long as it's necessary for the plot and not too unbelievable.
In the end I give this episode of MythBusters an A-. The only thing I think it as missing was a better treatment of what HF can actually do. They did an excellent job of explaining acid chemistry, which was nice.
Podcast: Bad Science in the Movies
Hello! Last week Sam and I recorded a podcast with the great Dr. Oh from Just Like Cooking. I'm calling the episode "Me-sa Love Lightsabers: Science in the Media". Enjoy!
Show notes:
Topic: Science in the Media
Panel:
Chad Jones
Sam Matthews
See Arr Oh
Articles mentioned:
The "Finding Nemo" Article
Time stamps on the way...
Show notes:
Topic: Science in the Media
Panel:
Chad Jones
Sam Matthews
See Arr Oh
Articles mentioned:
The "Finding Nemo" Article
Time stamps on the way...
Monday, August 5, 2013
Organometallic Isomers, Awesome Colors, and Agostic Interactions
There are basically only two reasons I decided to study chemistry: Color changes and explosions. Explosions held my interest for a short amount of time, but color changes have always been fascinating to me. I love watching clock reactions like the . So of course I was fascinated by this recently published work.
Published recently in the chemistry journal Angewandte Chemie International (a journal that until 6 months ago I couldn't even pretend to know how to pronounce) is the paper "Isolation of Two Agostic Isomers of an Organometallic Cation: Different Structures and Colors", in which Bullock et al. report a system with some very interesting chemistry.
Before reading this article I hadn't heard of agostic interactions (or I had heard and promptly forgotten), but they really are my kind of chemistry. I love weak interactions. An agostic interaction is one in which a caron-hydrogen bond interacts with a transition metal. These interactions are very weak (<15 kcal/mol), and that's really what makes this paper so surprising to me.
The isolation of two different isomers whose crystals are different colors isn't anything new. We've known for a long time that transition metals create some interesting color varieties. What's interesting in this paper is how such a small change can create such a large difference. The two conformations are very similar - the only difference being a C-C rotation and a different hydrogen forming the agostic interaction.
Computationally (DFT) the energy differnece between these two conformations is only 0.6 kcal/mol, an extremely small amount. It's enough, however, to produce a very different color. The small change in conformation produces a much larger change in the HOMO/LUMO transition. To me the interesting chemistry here isn't that a violet solution precipitates out both blue and orange crystals (but again, color changes are neat). The interesting chemistry is that very, very subtle changes in this system's conformation lead to such distinct color differences. Colors that are actually at the opposite ends of the visible spectrum.
 van der Eide EF, Yang P, & Bullock RM (2013). Isolation of Two Agostic Isomers of an Organometallic Cation: Different Structures and Colors. Angewandte Chemie (International ed. in English) PMID:
van der Eide EF, Yang P, & Bullock RM (2013). Isolation of Two Agostic Isomers of an Organometallic Cation: Different Structures and Colors. Angewandte Chemie (International ed. in English) PMID:

|
|
Two Crystals: Both alike in conformation in fair PNNL, where we lay our scene Image Credit: PNNL |
Published recently in the chemistry journal Angewandte Chemie International (a journal that until 6 months ago I couldn't even pretend to know how to pronounce) is the paper "Isolation of Two Agostic Isomers of an Organometallic Cation: Different Structures and Colors", in which Bullock et al. report a system with some very interesting chemistry.
Before reading this article I hadn't heard of agostic interactions (or I had heard and promptly forgotten), but they really are my kind of chemistry. I love weak interactions. An agostic interaction is one in which a caron-hydrogen bond interacts with a transition metal. These interactions are very weak (<15 kcal/mol), and that's really what makes this paper so surprising to me.
The isolation of two different isomers whose crystals are different colors isn't anything new. We've known for a long time that transition metals create some interesting color varieties. What's interesting in this paper is how such a small change can create such a large difference. The two conformations are very similar - the only difference being a C-C rotation and a different hydrogen forming the agostic interaction.

|
| The difference is highlighted in red. It's not much, really. Image Credit: Cited paper |
Computationally (DFT) the energy differnece between these two conformations is only 0.6 kcal/mol, an extremely small amount. It's enough, however, to produce a very different color. The small change in conformation produces a much larger change in the HOMO/LUMO transition. To me the interesting chemistry here isn't that a violet solution precipitates out both blue and orange crystals (but again, color changes are neat). The interesting chemistry is that very, very subtle changes in this system's conformation lead to such distinct color differences. Colors that are actually at the opposite ends of the visible spectrum.
The work in this paper seems very well done. DFT calculations (including HOMO/LUMO analysis), X-ray crystallography, and IR spectra all agree with the conclusion that this simple bond rotation creates such a stark difference in the properties of these crystals.
Periodic Table Trends
I just finished writing an article for answers.com about trends in the periodic table, a topic I'm obviously comfortable with (and many of the readers of this blog are as well). To be honest I checked the trends with wikipedia and other sources many, many times - worried that somehow I had forgotten them.
It was a chance to rethink about the periodic table, though, which was fun. Mendeleev's work is pretty impressive. Being able to predict new elements the way he did was amazing (and will most likely be the subject of a future article).
So tell me, readers, What do you think is most impressive about the periodic table? What is outdated or needs changed? Do you prefer any of the "alternate" forms of the periodic table?
It was a chance to rethink about the periodic table, though, which was fun. Mendeleev's work is pretty impressive. Being able to predict new elements the way he did was amazing (and will most likely be the subject of a future article).
So tell me, readers, What do you think is most impressive about the periodic table? What is outdated or needs changed? Do you prefer any of the "alternate" forms of the periodic table?
Friday, August 2, 2013
My first post for Answers.com: Do's and Don'ts of Learning Chemistry
As you may have already seen, I'm the new Category Expert Writer for chem.answers.com. My first article is now published:
I've taught several hundred freshmen in my short time as a graduate student. Without fail they make the same mistakes - even if I tell them otherwise. This list is basically the advice I give to my new students.
So tell me, internet chemistry friends, What advice do you give? Is there any advice that you give but know it will always be ignored?
The Do's and Don'ts of Learning Chemistry
I've taught several hundred freshmen in my short time as a graduate student. Without fail they make the same mistakes - even if I tell them otherwise. This list is basically the advice I give to my new students.
So tell me, internet chemistry friends, What advice do you give? Is there any advice that you give but know it will always be ignored?
Heading in a new direction: The future of The Collapsed Wavefunction
For the past two years The Collapsed Wavefunction has been a major part of my identity. I've devoted hours of my time to writing about chemistry, physics, math, biology, critical thinking, bad science in the movies, and pretty much anything else that interested me.
A few weeks ago I was contacted by a recruiter from answers.com. They offered me a position as a Category Expert, which I have now officially accepted. You can now find me at chem.answers.com
What does this mean for The Collapsed Wavefunction
I have loved writing and managing my own blog. It gave me the freedom to say whatever I wanted to whenever I wanted to. Accepting a "real" writing job means that I now have deadlines, content restrictions, style expectations, and the list goes on and on. This means that I won't have as much time to write The Collapsed Wavefunction (come on, guys, I'm trying to get a PhD on top of all of this!) but you haven't seen the last of me. The Collapsed Wavefunction will still be publishing new articles and the podcast has only just begun.
With each post I write for chem.answers.com I plan on publishing a follow-up here on The Collapsed Wavefunction. It may only be a link to the article but let's face it - I've always been a pretty sarcastic writer. Keep following The Collapsed Wavefunction for bonus content!
Thank you to everyone that reads my silly little science blog. Being involved with such an awesome community has made this hobby very fulfilling.
A few weeks ago I was contacted by a recruiter from answers.com. They offered me a position as a Category Expert, which I have now officially accepted. You can now find me at chem.answers.com
What does this mean for The Collapsed Wavefunction
I have loved writing and managing my own blog. It gave me the freedom to say whatever I wanted to whenever I wanted to. Accepting a "real" writing job means that I now have deadlines, content restrictions, style expectations, and the list goes on and on. This means that I won't have as much time to write The Collapsed Wavefunction (come on, guys, I'm trying to get a PhD on top of all of this!) but you haven't seen the last of me. The Collapsed Wavefunction will still be publishing new articles and the podcast has only just begun.
Don't leave me! I'm still here!
With each post I write for chem.answers.com I plan on publishing a follow-up here on The Collapsed Wavefunction. It may only be a link to the article but let's face it - I've always been a pretty sarcastic writer. Keep following The Collapsed Wavefunction for bonus content!
Thank you to everyone that reads my silly little science blog. Being involved with such an awesome community has made this hobby very fulfilling.
Subscribe to: Posts (Atom)

