Newest podcast episode is up!
Topic: Our Favorite Scientists
Panel: Just Sam and I
Music by Jonathan Coulton
Relevant links:
http://www.smbc-comics.com/?id=2260 - The SMBC comic mentioned
Monday, July 29, 2013
Friday, July 26, 2013
Bad Science in the . . . Magic School Bus?
Ok, I like to write about bad science that I see in the movies. I think it's fun to use movies to segue into a real discussion about science. Today I was watching The Magic School Bus with my kids and, as usual, I started over-thinking everything. I realize, of course, that The Magic School Bus isn't supposed to be scientifically accurate. It's a way to introduce kids to science (or rather, scientific facts) in a way that keeps them entertained. That being said, I couldn't get this thought out of my head: When the students shrink what happens to their internal organs?
 |
| Ms. Frizzle: An administrative nightmare |
More specifically I was wondering how blood transportation would work. Here's how normal respiration works:
When you breath in O2 it enters your lungs and makes its way into the alveoli - small pockets in your lungs that fill up with gas (ideally oxygen). There's a lot of interesting gas dynamics that happen here, but I'll keep it short by saying that O2 is transported from the gas phase (lungs) into liquid phase (blood).
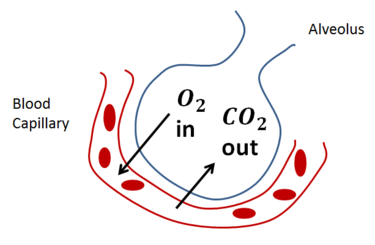

 |
| The alternating red and blue colors are to help you visualize the different binding areas. They're really all the same. Image credit: Wiki commons |
So, if Ms. Frizzle's class shrinks down to investigate a flower, bake a cake, or investigate the insides of one of their classmates, the hemoglobin must shrink with them. The binding between oxygen and hemoglobin depends on their size similarities, so when they breath in oxygen it would be unable to bind to the hemoglobin in their body; Asphyxiation would be the immediate result of shrinking. This is the same story with Honey I shrunk the kids. Being small make it impossible to breath.
The only explanation I can think of is maybe there's some kind of school bus magic that shrinks the oxygen down as they inhale. This would actually help explain another problem - how does Arnold suffer from pollen allergies when he's smaller than pollen? If the pollen around him shrinks as he inhales then being small is no cure for his allergy woes. This explanation brings new problems with it, though. If the oxygen shrinks to enter your lungs then it takes up less space (obviously). This means that the pressure gradient between outside your body and inside your lungs changes, allowing more oxygen to flood in - and shrink again. It seems to me that oxygen would flood in your lungs until they burst.
| Hmmm . . . I really didn't think this would end this morbidly. |
Of course if we're talking about a Magic school bus I guess "magic" is always a valid answer, right?
Monday, July 15, 2013
Punching down? I don't remember swinging at all.
Over the last few weeks there have been several good blog posts written about chemophobia. I suppose this latest round of #Chemophobia began with the now infamous article (at least in the chemistry world) posted on BuzzFeed - "8 foods we eat in the US that are banned in other countries". Sam Matthews wrote an article for this blog, pointing out some of the same bad science and, of course, Derek Lowe gave a much more detailed debunking soon after. Ash Jogalekar suggested a chemical lobbying organization - he's calling it the National Center for Chemical Education. Chris Clarke, over at Pharyngula, apparently thinks we're all douchebags for making the lame "dihydrogen monoxide" joke. Granted, I don't think he's talking about me. When I have made the joke (which I'll admit is pretty lame) I've made it to a group of chemists, so that can't be considered "punching down" can it? Soon after that post came two great articles defending chemists - one found at Behind NMR Lines and another at Doing Good Science.
The question I'm left with is this: What is the right way to fight chemophobia?
I certainly don't feel like I've been punching down. I don't spend my time on the internet to mock someone who doesn't have the same education that I do. I'm not trying to get in the "smart kid's club" by proving that I know a lot about chemistry. In fact I've been very upfront - I don't think I'm incredibly special or inherently smart. For this very reason I think science - to truly be science - must be clear and easily communicated. That doesn't always mean it will be easy to master, but science is designed to simplify, not complicate, our universe. It is the universe - not science - that is hard to understand.
Onto chemophobia, then. How do we tell someone they are wrong without making them feel wrong. Someone that feels wrong is less likely to accept what you have to say - you haven't countered their argument you've insulted them. Accepting that you're right means accepting your insult. I think it's important, and I've said it before, that we don't belittle someone that is most likely just misinformed.
That's why I don't think the "but everything is a chemical" argument is very persuasive - someone using the word chemical instead of toxin isn't using the word chemical in a "everything is a chemical" kind of way. If you're convinced that chemical always means "all matter in the universe" take a look at this picture:
The question I'm left with is this: What is the right way to fight chemophobia?
I certainly don't feel like I've been punching down. I don't spend my time on the internet to mock someone who doesn't have the same education that I do. I'm not trying to get in the "smart kid's club" by proving that I know a lot about chemistry. In fact I've been very upfront - I don't think I'm incredibly special or inherently smart. For this very reason I think science - to truly be science - must be clear and easily communicated. That doesn't always mean it will be easy to master, but science is designed to simplify, not complicate, our universe. It is the universe - not science - that is hard to understand.
Onto chemophobia, then. How do we tell someone they are wrong without making them feel wrong. Someone that feels wrong is less likely to accept what you have to say - you haven't countered their argument you've insulted them. Accepting that you're right means accepting your insult. I think it's important, and I've said it before, that we don't belittle someone that is most likely just misinformed.
That's why I don't think the "but everything is a chemical" argument is very persuasive - someone using the word chemical instead of toxin isn't using the word chemical in a "everything is a chemical" kind of way. If you're convinced that chemical always means "all matter in the universe" take a look at this picture:

|
| Thanks to for this photo |
Unless you can look at this picture and honestly say that Wal-Mart has correctly labeled their brooms and mops you must admit that "chemical" can have more than one meaning. Colloquially it means something closer to "toxin" or "harsh cleaner" or "industrial additive". You get the idea. It usually does mean something that you don't want to ingest.
Often when I see #chemophobia on Twitter it really just comes down to a vocabulary misunderstanding. If the only problem with an article is the misuse of the word chemical, then maybe we should let it go. Perhaps we, as chemists, are a little too protective of our namesake. If the public understands chemical to mean "toxin" then we need to acknowledge that - not mock it. Go ahead and point out that everything is a chemical (by your definition at least), but don't let that be the end of your argument. Continue on to point out why the specific chemical in question is not bad for you (or whatever the case may be).
It's a bit of tightrope that we walk, though, and sometimes we over-correct. Let's face it, chemicals can be very bad for you. Sometimes, in the midst of our #chemophobia rage we seem to be forgetting that - or at least trying to ignore it. Most of the things that the public labels as "chemicals" probably are toxins. My guess is that Wal-Mart, in the picture above, meant to stock bleach, ammonia, and other cleaners under that sign. I wouldn't suggest drinking any of those. We say nothing when ammonia is called a chemical (because, well, it is a chemical). The word chemical, then, becomes associated with bleach, ammonia, and industrial strength solvents. That association makes it difficult to teach someone that aspartame is a chemical but you can drink a diet soda just fine. Chemical is already so well connected with toxin that it's not always an easy task.
I think we (chemists) really are in a bit of a tight spot. Ash may be onto something - we really might need to start up the National Center for Chemical Education. I don't think it's a simple solution, but it may be the right solution.
But whatever we decide to do just stop throwing punches, ok?
Tuesday, July 9, 2013
Podcast Episode #1 - GMOs
Here you go! Official episode #1 of the podcast. Sam and I spoke with Kevin Bonham, a Harvard graduate student, about genetically modified organisms. Enjoy!
Please, if you use iTunes please subscribe and leave a rating for the show!
Articles cited, talked about, or used for our own research in making this episode:
- http://blogs.discovermagazine.com/science-sushi/2013/06/19/the-very-thick-line-between-raising-concerns-and-denialism#.UcKhZFV7P2F
- http://www.mpg.de/7023230/W001_Viewpoint_012-016.pdf
- http://www.npr.org/blogs/thesalt/2012/10/18/163034053/top-five-myths-of-genetically-modified-seeds-busted
- http://news.nationalpost.com/2013/01/26/the-myth-of-indias-gm-genocide-genetically-modified-cotton-blamed-for-wave-of-farmer-suicides/
- http://blogs.scientificamerican.com/guest-blog/2013/05/30/allergic-to-science-proteins-and-allergens-in-our-genetically-engineered-food/
Thursday, July 4, 2013
The flame test and fireworks
An important skill for chemists is being able to identify an unknown chemical or mixture of chemicals. Modern chemists have a myriad of tools available to help them do this. Much of modern chemical identification can be done in a "black box" way - inject a sample into a box, press start, and get your answer. We have instruments like IR Spectroscopy, GC-MS, and HPLC to help us determine what is present in a solution. Technology like we have today certainly wasn't available to early chemists, but they did have other fun chemical tests to help.
One of the earliest chemical tests was the flame test. It was known that a flame will burn different colors depending on the chemicals present long before we had the theory to explain it. When lithium is present, for example, a flame will burn bright red. Barium will burn green. To understand why we needed to first develop quantum mechanics. An electron is excited (its energy increases) by a flame. After staying in an excited state for some time the electron will eventually relax. When it does it releases a photon. The energy, and therefore color, of the photon depends on the element involved. This simple test can therefore tell us a great deal of information about what the chemical make-up of a solution is.
The modern application is, of course, fireworks. When fireworks are made they are stuffed with different chemical elements. Each element giving a distinct color. So tonight, when you see fireworks don't just say "Ooohh....Ahhhh" say "Oooohhh copper....Ahhh...sodium".
Here's a list of the chemicals used for fireworks:
Red: strontium or lithium salts
Orange: calcium salts
Gold: iron
Yellow: sodium sals
Green: barium salts
Blue: copper salts
Purple: mixture of strontium (red) and copper (blue) salts
White: magnesium or aluminum
And this great video about what makes fireworks "pop"
One of the earliest chemical tests was the flame test. It was known that a flame will burn different colors depending on the chemicals present long before we had the theory to explain it. When lithium is present, for example, a flame will burn bright red. Barium will burn green. To understand why we needed to first develop quantum mechanics. An electron is excited (its energy increases) by a flame. After staying in an excited state for some time the electron will eventually relax. When it does it releases a photon. The energy, and therefore color, of the photon depends on the element involved. This simple test can therefore tell us a great deal of information about what the chemical make-up of a solution is.
The modern application is, of course, fireworks. When fireworks are made they are stuffed with different chemical elements. Each element giving a distinct color. So tonight, when you see fireworks don't just say "Ooohh....Ahhhh" say "Oooohhh copper....Ahhh...sodium".
Here's a list of the chemicals used for fireworks:
Red: strontium or lithium salts
Orange: calcium salts
Gold: iron
Yellow: sodium sals
Green: barium salts
Blue: copper salts
Purple: mixture of strontium (red) and copper (blue) salts
White: magnesium or aluminum
And this great video about what makes fireworks "pop"
Subscribe to: Posts (Atom)
