How it Works:
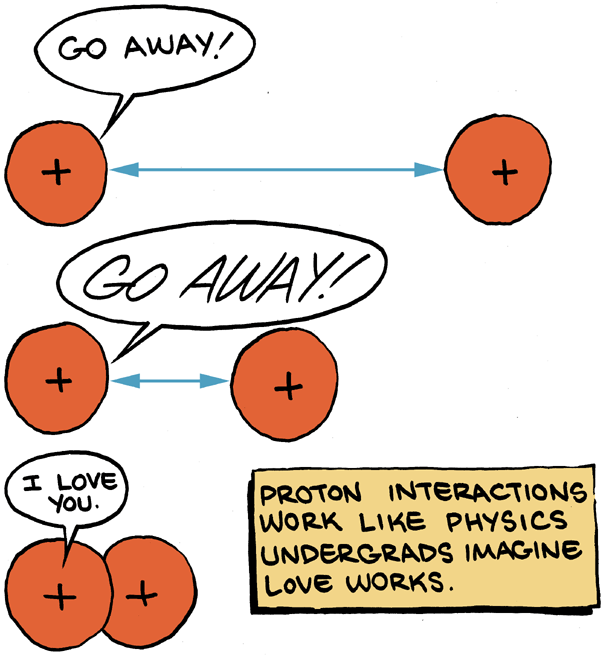
You probably remember from a physics class that there is positive charge and negative charge. Things of different charge attract, while things of like charge repel. As it happens, the strength of that force of attraction or repulsion follows an inverse square law. That simply means that the force is proportional to 1/r
2, where are is the distance between charged things. So, if the distance is 1000 meters, the force will be very small. If the distance is .001 meters, the force will be very strong.
You may also have heard that it’s possible to fuse nuclei. But according to that inverse square law (which, when you add in a bunch of constants is called Coulomb’s Law), if you get those positively
charged nuclei next to each other, the distance basically drops to 0. This should present a problem, partially because we all know that when you divide by 0 the universe ends, but more importantly, shouldn’t all nuclei be exploding right now?
That’s where something called Strong Nuclear Force comes in. Strong nuclear force is a fundamental force, the same as gravity or electromagnetism. It is very, very powerful, but it only acts over very short distances. So, you don’t really interact with it very much, at
least as far as you know.
So, in order to fuse two atoms (for simplicity, let’s say two hydrogen atoms) you’ve got to get those nuclei really, really close - close enough that the Strong Force of attraction overcomes the Coulomb Force of repulsion. This is called the “Coulumb Barrier.” Simply put, if you want fusion, you need two atoms to go really fast as they smack into each other.
Getting it to Happen
You can actually do it on your tabletop, using what’s called a Farnsworth Fusor. It’s basically an electrified cage that zaps protons toward its middle. Although most of the protons will not fuse, a few will randomly happen to have a bunch of energy, and will hit each other hard enough to fuse.
Think about it like this - if you have a million superballs bouncing in a zero gravity container, they’ll keep trading energy as they bounce around. Occasionally, one super ball will get hit on its backside repeatedly, causing it to speed way up. If another ball does the same and they happen to hit each other,
BOOM! Okay, the analogy fails there because the superball will just explode. At the atomic scale, there’s a chance the Strong Force will kick in and make them stick together.
The way we know fusion has happened (typically) is by detecting energetic neutrons. Long story short - when fusion happens, sometimes a neutron gets squeezed out of the new nucleus at very high energy. You’re unlikely to encounter one of these during your day, so if you’re detecting a bunch coming out of your fusor, you know you’re in business. This is why, when people claim they have discovered cold fusion, scientists usually say “show us your neutrons!”
The Problem
So how come we don’t have these in every home? Well, although you can get fusion to happen on your tabletop, it costs energy. It takes more energy to get those protons to sit in the middle of the cage than you could possibly get back by, for example, having those neutrons slam into some water and heating it to power a steam turbine.
But, we know fusion works. It happens in the core of the sun all the time. Unfortunately, it works there because of the massive gravity of the sun, which confines vast amounts of hydrogen in its center. Until someone figures out how to generate artificial gravity (hopefully without destroying Earth in the process) we can’t do that here on terra firma.
Possible Solutions
Ever since fusion was discovered, scientists and engineers have tried to figure out a way to use it as an energy source. The various methods are outside the scope of this article, but here are a couple ways you could possibly pull off the trick:
1) Pinch it - Using devices like tokamaks (basically a donut-shaped version of the fusor), and very strong electromagnetic force, you can crunch the protons into a small space. They kick out neutrons, which you use for power.
2) Blast it - In most cases this involves lasers. You can confine the protons to a small container, then zap it from a bunch of angles at once. This confines the protons into a small space to hopefully get you some more energy out than you put in.
3) Hybrid - One recent development at Sandia labs is called MagLIF, short for Magnetized Linear Inertial Fusion. Basically, you use a laser to heat up the small container , then you use a powerful magnetic force to crunch the container down (first you blast it, then you pinch it!).
Current State
So far, nobody has achieved “energy gain.” That is, nobody has gotten more energy out than was put in. And, this is not for lack of trying. Fusion has turned out to be a difficult problem, which has caused the unfortunate truism that “fusion is always 50 years away.”
However, there are a number of active projects, doing all of the above approaches. There are also a number of heterodox approaches that are longshots, such as polywell fusion and focus fusion. Although it hasn’t happened yet, as the science improves, and we get bigger and bigger machines, like the Z Machine at Sandia and the forthcoming ITER tokamak in France, we can have reasonable hope that energy gain will happen in finite time.
Why It Would be Great
Fusion power is essentially limitless. The usual fuel for fusion reactions is deuterium, which is an isotope of hydrogen. It is abundant enough in the ocean that, even though we aren’t all running fusion reactors, you can actually
buy it (relatively) cheap online.
If fusion did work, and could be made inexpensive enough, it’d mean limitless clean energy at low cost. Although most reactor designs produce some irradiated material (the walls of the confinement chamber, for example), it tends to be in smaller quantities and with a lower half life than standard fission reactors. As society relies more and more on energy for everything, lowering the cost of energy means far more abundance. Everything from laptops to chemical fertilizer requires energy input. A number of crucial services, like desalinization, also require a lot of energy. If that energy could be made very cheap, many problems social and environmental could be solved.
In fact, it’s hard to think of problems that wouldn’t be lessened by nearly free energy. For anything you own or any service you purchase, somewhere along the chain, a power plant is involved. Imagine how much it would change your world if both the financial and environmental cost of the things you desire were minimized.
 Becoming a skilled surgeon is no easy task, but new research suggests that surgeons may be able to have a little bit of fun while they train. In a paper published today in PLOS One, researchers show have shown that a training regimen including the Wii can improve surgical skills.
Becoming a skilled surgeon is no easy task, but new research suggests that surgeons may be able to have a little bit of fun while they train. In a paper published today in PLOS One, researchers show have shown that a training regimen including the Wii can improve surgical skills.