I'm subscribed to many science related discussion boards. Many of them on Facebook. I don't know why, really. They're full of internet trolls and bad science. So, in an effort to correct some misconceptions that I've seen (and because someone is wrong on the internet), I'm adding a segment I'm calling:
Someone is wrong on the internet
Today's post isn't necessarily someone that is wrong, but someone that presented their info in a very confusing way. Here's what I saw today on Facebook:
I'm sure you could make some pedantic correction somewhere, but to my understanding this is all correct.
However. . .
This was written on a page intended for a general audience. I had to read through it carefully to check the information, so in no way was this posted to the right audience. In fact, the very first comment says
"Good info. Bt what is d-orbital ?"
Which is an obvious sign that the message wasn't understood. So, because someone is wrong on the internet, I now present you with my reexplanation of crystal field theory (as it should be presented to this audience):
Crystal Field Theory
When you think of electrons, you probably think of them as small, highly charged, bouncy balls. Sometimes this is a great way to think about them. However, like all matter, electrons will sometimes act like particles and sometimes act like waves.
Now, that guy was talking about orbitals. What are orbitals, and what is a d-orbital? Orbitals are the mathematical way that we describe the properties of electrons within an atom. An orbital is where an electron "lives". Electrons don't "orbit" the nucleus, they exist everywhere around the nucleus in what we call an "orbital" (but remember, nothing is orbiting).
The shape of an orbital is described by the l quantum number (that's a lower case "L", by the way). l can only equal 0,1,2,3... It can't equal 2.3 or 1.5 or 5.43 or anything that isn't an integer number. This is the quantum part of quantum mechanics. Things can only exist in specific energy levels.
We have chosen to name the orbitals as follows:
l = 0, which we call an "s"-orbital. There is only one, and it looks like this:
l = 1, which we call the "p-orbital". There are three, and they all look like this:
l = 2, which we call the "d-orbital". There are five, and they look like this:
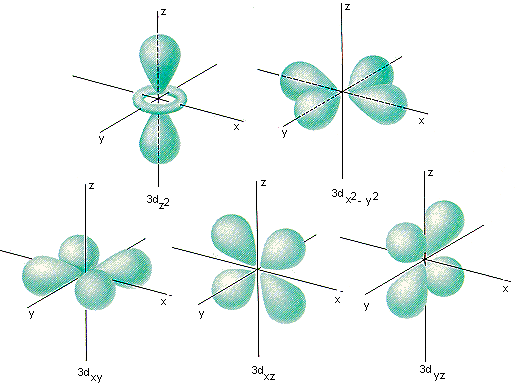
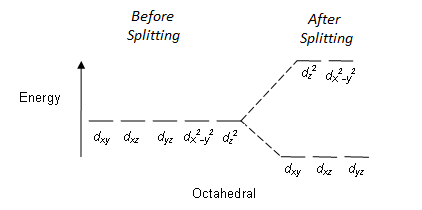
I'll stop here, since we've reached the orbitals important to crystal field theory (if you'd like to know how to draw all of those yourself check out my post here). Now, in theory each of these d-oritals have the same amount of energy. However, in practice we have found that sometimes that's not true. Sometimes when an ion (a charged atom or molecule) binds to a transition metal (Cobalt, for example) the ion will split the energy of the d-orbitals.
Notice that before the splitting all 5 orbitals had the same energy. After splitting, though, three orbitals are lower in energy and two are higher. The differnce in energy between these energy levels is what creates the amazing colors in transition metal complexes. As the electrons "jump" between those energy levels they release energy as light and we're left with pretty colors!