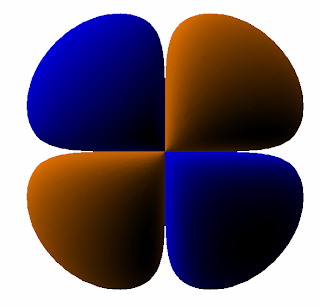I've been teaching general chemistry concepts for several years now. For some reason the idea of atomic orbitals is hard for students to grasp. Tonight I'm holding an exam review for my students and I thought I'd prepare by writing about the quantum numbers and how they affect the shape of atomic orbitals.
The Quantum Numbers
n: The principal quantum number. n can be any positive integer (1,2,3,4...)
l: The angular momentum quantum number. l can equal: 0,1,2,3,4...(n-1)
When we write the angular quantum number we will often use the notation:
0 = s1 = p
2 = d
3 = f
There are two other quantum numbers, but we aren't concerned with those for this exercise.
Nodes
In quantum mechanics a node is a point where an electron cannot exist. The shape of any orbital is defined by the nodes. There are two types of nodes: angular and radial.
Angular: An atomic orbital will have l angular nodes. So, an s-orbital has 0 a p-orbital has 1, and so on.
Radial: Each atomic orbital will have n-l-1 radial nodes. So a 3p orbital has 3-1-1 = 1 radial node.
Drawing orbitals
Ok, so what you've read above is explained in just about every general chemistry textbook. But, when asked to draw orbitals most chemists just pull from memory. You don't need to do that, though. You don't have to memorize the shape of a single atomic orbital. You just need to know understand the quantum numbers. Let's start with 1s.
1s
Angular nodes: 0
Radial nodes: 0
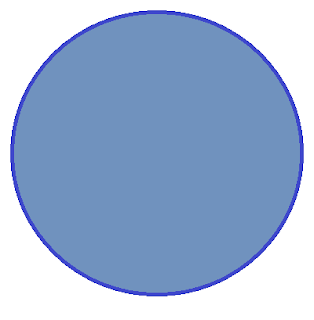
This is the easiest. We'll always start with a circle. There are zero nodes and so we're already done. We'll say that 1s looks like this:
Moving on...
2p
Angular nodes: 1
Radial nodes: 0
We start with the same circle, but this time we need to add an angular node.
Which, once you smooth the edges looks just like the actual 2p orbital:
Let's do the 3d orbital now
3d
Angular nodes: 2
Radial nodes: 0
Which, after we smooth out the edges, is a pretty good prediction:
The great thing about drawing the atomic orbitals this way is you never have to memorize any of these shapes - they come directly from understanding what the quantum numbers mean.
Now, if you're one of my students, get back to studying.
Notes
All of the "real" atomic orbitals from above were created using Orbital Viewer, a very cool (and free) program.
Nodes
In quantum mechanics a node is a point where an electron cannot exist. The shape of any orbital is defined by the nodes. There are two types of nodes: angular and radial.
Angular: An atomic orbital will have l angular nodes. So, an s-orbital has 0 a p-orbital has 1, and so on.
Radial: Each atomic orbital will have n-l-1 radial nodes. So a 3p orbital has 3-1-1 = 1 radial node.
Drawing orbitals
Ok, so what you've read above is explained in just about every general chemistry textbook. But, when asked to draw orbitals most chemists just pull from memory. You don't need to do that, though. You don't have to memorize the shape of a single atomic orbital. You just need to know understand the quantum numbers. Let's start with 1s.
1s
Angular nodes: 0
Radial nodes: 0
This is the easiest. We'll always start with a circle. There are zero nodes and so we're already done. We'll say that 1s looks like this:
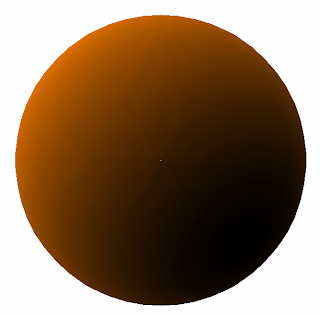
And sure enough, the 1s orbital looks like we predicted. Here's the 1s orbital:
But now let's move on.
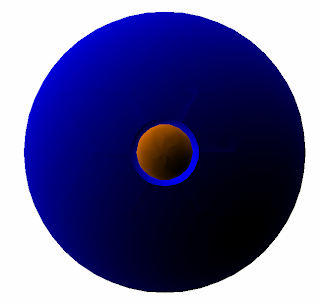
2s
Angular nodes: 0
Radial nodes: 1
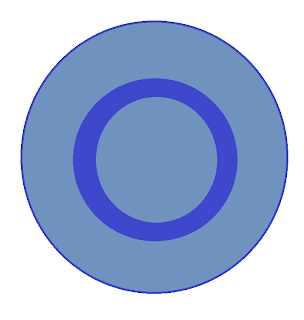
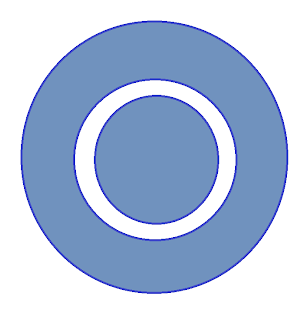
We start again with a circle, but we draw one smaller circle within the larger circle.
We erase everything in that node and this is now our prediction for what a 2s orbital will look like.
And sure enough, our prediction is pretty close. Here's the real 2s orbital:
Moving on...
2p
Angular nodes: 1
Radial nodes: 0
We start with the same circle, but this time we need to add an angular node.
When we erase that section we are left with our prediction for a 2p orbital...
Which, once you smooth the edges looks just like the actual 2p orbital:
Let's do the 3d orbital now
3d
Angular nodes: 2
Radial nodes: 0
Following the same process we have marked two angles through the center of the circle. When we erase that area we're left with our prediction for a 3d orbital:
Which, after we smooth out the edges, is a pretty good prediction:
Let's do one more (because I'm not quite sick of this yet)
3p
Angular nodes: 1
Radial nodes: 1
We start with an angular node...
But, once we erase that are we're left we still have to include the radial node...
So now let's just erase that area, and we'll be left with our prediction:
Which again, once we smooth out the edges, looks pretty close to the actual 3p orbital:
The great thing about drawing the atomic orbitals this way is you never have to memorize any of these shapes - they come directly from understanding what the quantum numbers mean.
Now, if you're one of my students, get back to studying.
Notes
All of the "real" atomic orbitals from above were created using Orbital Viewer, a very cool (and free) program.