The Chemical: Cucurbit[n]uril
Systematic name: Dodecahydro-1H, 4H, 14H, 17H-2, 16:3, 15-dimethano-5H, 6H, 7H, 8H, 9H, 10H, 11H, 12H, 13H, 18H, 19H,20H, 21H, 22H, 23H, 24H, 25H, 26H-2, 3, 4a, 5a, 6a, 7a, 8a, 9a, 10a, 11a, 12a, 13a, 15, 16, 17a, 18a, 19a, 20a, 21a, 22a, 23a, 24a, 25a, 26a-tetracosaazabispentaleno[1’’’, 6’’’:5’’, 6’’, 7’’]cycloocty[1’’, 2’’, 3’’:3’,4’]pentaleno (1’, 6’:5, 6, 7) -cycloocta (1, 2, 3-gh:1’, 2’, 3’-g’h’) cycloocta (1, 2, 3-cd:5, 6, 7-c’d’) dipentalene-1, 4,6, 8, 10, 12, 14, 17, 19, 21, 23, 25-dodecone
You probably know it as: Well...you probably don't know it.
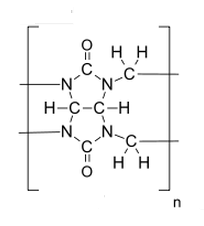
The structure:

Cucurbit[n]urils, or CB[n] for short, are shaped sort of like a pumpkin, which is actually how they get the name (pumpkins belong to the family cucurbitaceae). They are, for lack of a better word, molecular cages - they can trap other molecules (we call trapped molecules "guests" and the CB[n] a "host"). They don't just bind to anything and everything, though. They're very selective about their binding (and it's not always easy to predict what will bind). For example, CB[7] will trap fluorescent dyes in its cavity. The dye won't fluoresce as long as it's in the cavity, but as soon as it's displaced it lights up. Phenylalanine will also bind to CB[7] and displaces the fluorescent dye. Some clever scientists realized that phenylalanine is found at the end of the insulin protein. They exploited this to create (or at least show a proof of concept for) a rapid analysis for insulin using CB[7].
Over and over cucurbiturils amaze me. They affect how molecules interact with light, they trick liquid phase molecules into thinking they're in the gas phase, they have been proposed for drug delivery, catalysis, waste management, and molecular architecture to name only a few applications. What's most amazing to me is how unpredictable each new derivative can be. With each new derivative that we study we find that the chemistry is vastly different, even when the compound itself is nearly the same (I'll insert some new results from my lab here once they're published). It's not a chemical you'll run into every day, but studying this chemical is what keeps me awake at night and gets me out of bed in the morning.