Now, before I continue on any further, I need to make something clear. Negative temperature is not below absolute zero. After all, how can something be colder than absolute zero? The truth may sound even stranger - negative temperature is actually on the scale of temperatures after infinity. So the scale of temperatures is:
+0 K, . . . , +300 K, . . . , +∞ K, −∞ K, . . . , −300 K, . . . , −0 K
I can't stress enough, though, that we're really not talking about temperature here in terms of "hot" and "cold". We're talking about the entropy definition of temperature. The mathematical description of temperature is:
Wolfgang Ketterle, a physicist and Nobel laureate at the Massachusetts Institute of Technology in Cambridge, has said of this work: It is "as though you can stand a pyramid on its head and not worry about it toppling over" - what exactly does that mean? To understand it, lets look at some energy level diagrams.
|
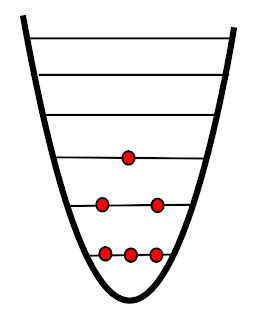
| "Normal" temperature |
The above diagram shows the quantum energy levels for some "normal" system. You'll notice that three of the particles are in the lowest energy level. As particles gain energy they are excited to higher levels. There are two in the first excited state, and one in the second excited state. If we describe entropy as the dispersal of energy you can see that as particles gain energy and are excited to higher levels the entropy increases - the particles are more "spread out".1
|
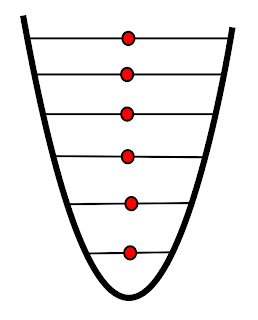
| Infinitely high temperature |
As temperature increases and the particles gain more and more energy they occupy more energy levels. Entropy will reach a maximum when the temperature is infinitely high.
|
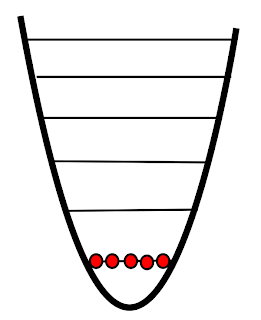
| Absolute zero |

|
| Negative temperature |
So how was this negative temperature reached? Well, to create a negative temperature you need to have an upper and lower bound to energy. Absolute zero is a lower bound to energy. You can't have a system with lower energy than absolute zero. The researchers needed an upper bound. But of course for a system near absolute zero there is nothing to stop energy from increasing - there's nowhere to go but up! Instead, they created an upper bound on energy by creating a optical lattice. Lasers arranged the potassium ions in such a way that there is an energetic barrier to the higher energy states. Thus, when energy is added to the system you get the inverted pyramid.
There are already plenty of ideas for applications of negative temperature. New quantum devices, heat engines with greater than 100% efficiency (without breaking the laws of thermodynamics), and even an explanation of dark matter. I don't see any of these as being realistic in the near future, though. What we gain from this right now is a better understanding of what temperature really is.
Notes
If you enjoyed this article like us on or follow on .
[1] I fully realize that this is not a rigorous definition of entropy, but it is useful definition. I'll be using it to explain negative temperature in a way that is hopefully helpful (and not too incorrect. Remember - all models are incorrect, some are just more useful than others)
If you enjoyed this article like us on or follow on .
