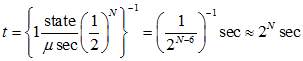"When sitting, your head is in the lower half of a room's volume. Estimate the frequency at which you will asphyxiate because all air molecules have spontaneously gone up to the higher half? Interpret the results."
Statistical Mechanics. My favorite (said with 0.0001% sarcasm). When I asked for homework problems I knew the day would come that I would be a bit stumped. I didn't know it would happen on the first submitted question. Thanks to @fxcoudert for the question. I hope I don't mess this up too much.
The first step in this question is to find the probability that all of the air molecules will be in one half of the room. Statistically this is simple; it's just:
So, partial credit at least. For this last part I know I have the right answer. At least correct to within a several orders of magnitude (and you'll see soon that a few orders of magnitude is close enough).
For the rest of the problem I'm going to be employing the ergodic hypothesis; I'm going to be assuming that over long periods of time the system will be in a particular state for a time that is equivalent to the probability of being in that state. So here's what we'll do. We start by putting all of the particles in a random position:
Then, after a certain amount of time we move all the particles to a new position:
The benefit of this approach is I can ignore any actual movement of the individual particles; we'll just assume that they move instantaneously from one state to another. This obviously doesn't happen in the real world but it's an assumption that is valid when you're looking at large numbers.
So how long is "a certain amount of time" that we leave between switching states? The best approach is probably to consider a state changed every time there is a collision. Depending on the pressure I'll estimate that at somewhere around one collision every microsecond. We can calculate how long it would take for all the particles to be in half of the volume by multiplying the time between changing states by the probability of that happening:
N is incredibly large (~1024). The age of the universe is ~1017 seconds. The amount of time it would take for all the molecules to be in the higher half is 21024 (and actually you wouldn't asphyxiate because they'd start moving away from each other to fill that empty space pretty quick, but I'll assume the question ignores that point).
Interpret the results? Well, you're safe to sit down. It won't kill you.